美国防晒法规(USA Sun Care Regulation)
FDA 认证的防晒剂(FDA Approved UV Filters)
非处方药(OTC )

指导准则(ICH Q7 GMP Guideline)

ICH Q7

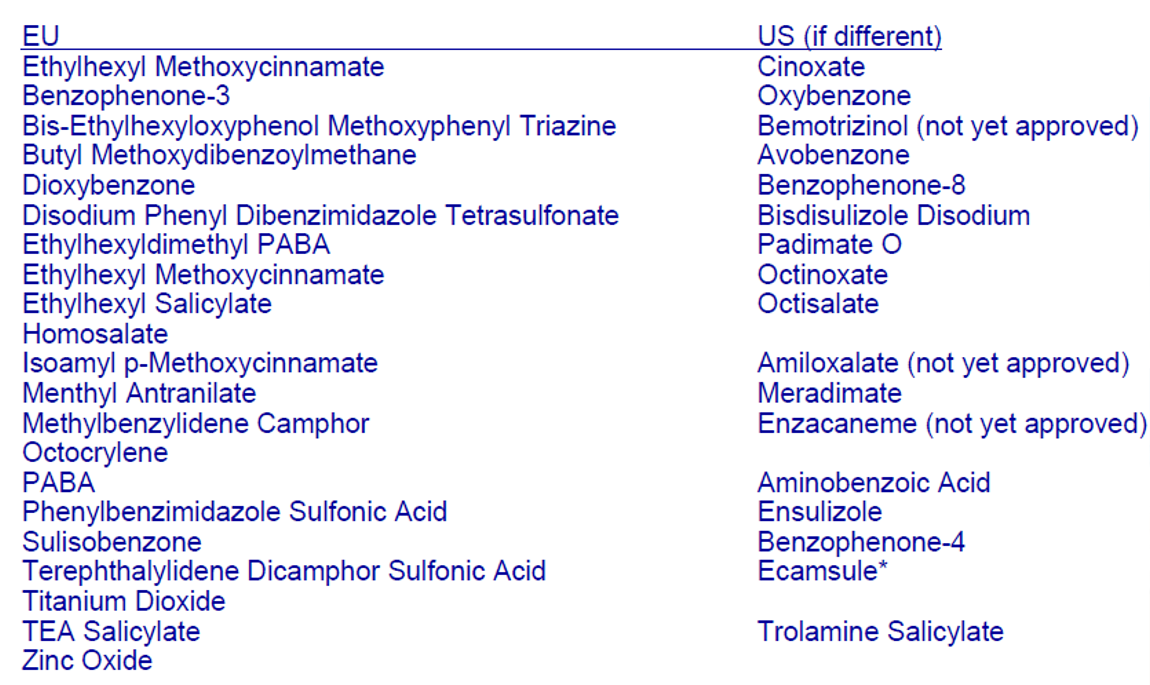
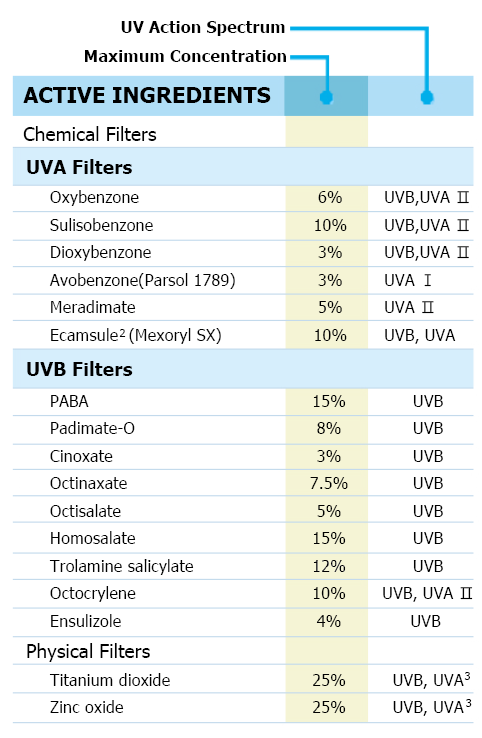
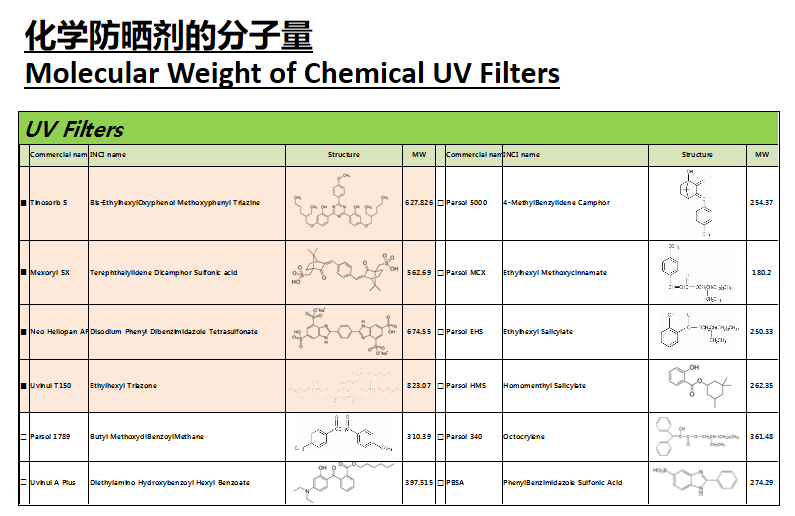
16种FDA 批准通过 可在美国使用的防晒剂(16 FDA approved UV filters for the US)
国家药品代码(NDC)
根据美国食品与药品管理局(FDA)的规定,产品的标签中印有“sunscreen”(防晒霜)或以任何方式表示或建议其用途是作为防止,缓解或治疗阳光射线带来影响的产品,定义为OTC(非处方)药物。 防晒霜(SUNSCREEN)应经由遵循OTC专论(OTC monograph)的企业生产、经销和销售。 以下是OTC专论(OTC monograph)的有关内容:
符合cGMP关于药物生产的法规。 标签标识符合非处方(OTC)药物的标签规定,包括药品信息标准标签。 生产商或经销商将最终的产品必须列在FDA的NDC的数据库中(国际药品代码名录) 推荐使用列在FDA的NDC名录中的防晒剂用到防晒霜产品中。
A product that includes the term “sunscreen” in its labeling or in any other way represents or suggests that it is intended to prevent, mitigate or treat the effects of solar radiation comes within the definition of a OTC(over-the-counter) drug according to the the US Food and Drug Administration (FDA). SUNSCREEN should be manufactured, distributed and sold followed by OTC monograph. Below are OTC monograph
Comply with cGMP regulations for drug manufacturing Labeling has to comply with labeling requirements for OTC drugs, including Drug Facts format labeling Final product has to be listed with the FDA's NDC database (National Drug Code Directory) by the manufacturer or the distributor Recommend to use UV filters in sunscreen that are listed with the FDA’s NDC directory
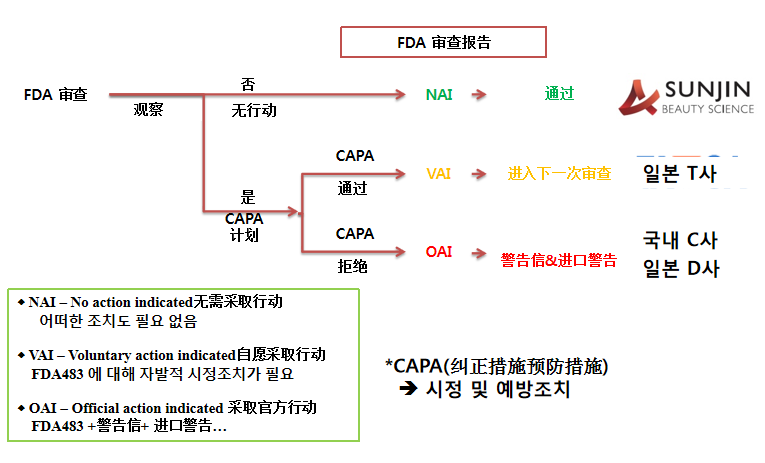
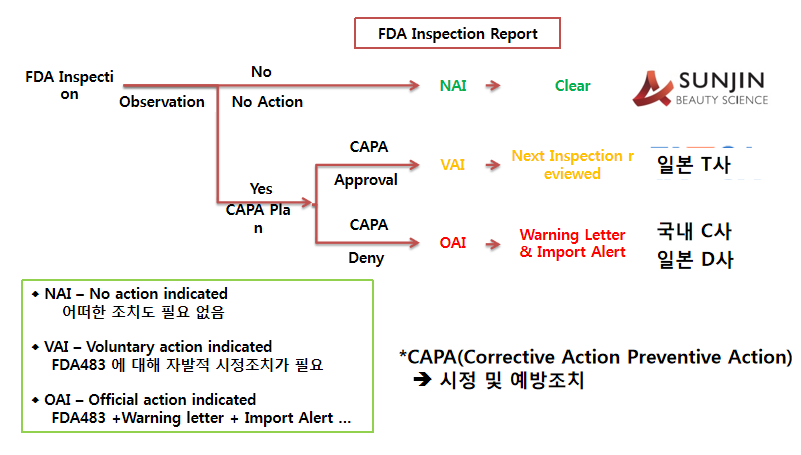
FDA审查流程(FDA Inspection Flow)
SUNJIN新工厂符合FDA标准@ Janghang, 2019.06(FDA Compliant New Factory @ Janghang, 2019.06)

FDA 审查报告(FDA Inspection Letter)
Inspection Site: Janghang Factory Inspection Date: 09/16/2019 – 09/19/2019 Inspection Result: “No action indicated” (‘NAI’). Based on this inspection, this facility is considered to be in an acceptable state of compliance with regards to current good manufacturing practice (CGMP).


NDC: National Drug Code (美国)国家药品代码
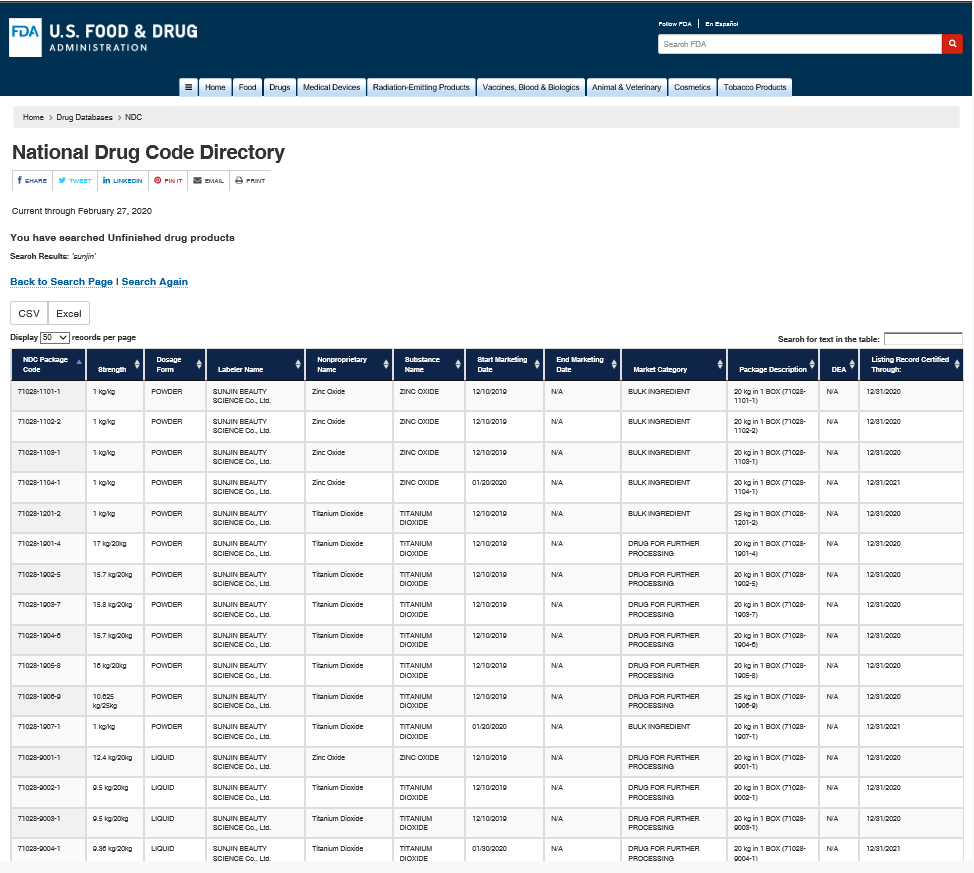
https://www.accessdata.fda.gov/scripts/cder/ndc/dsp_searchresult.cfm
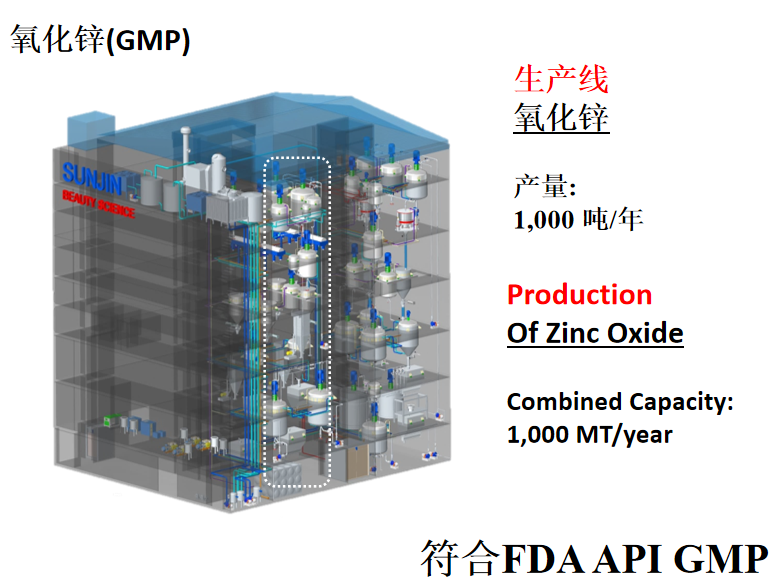
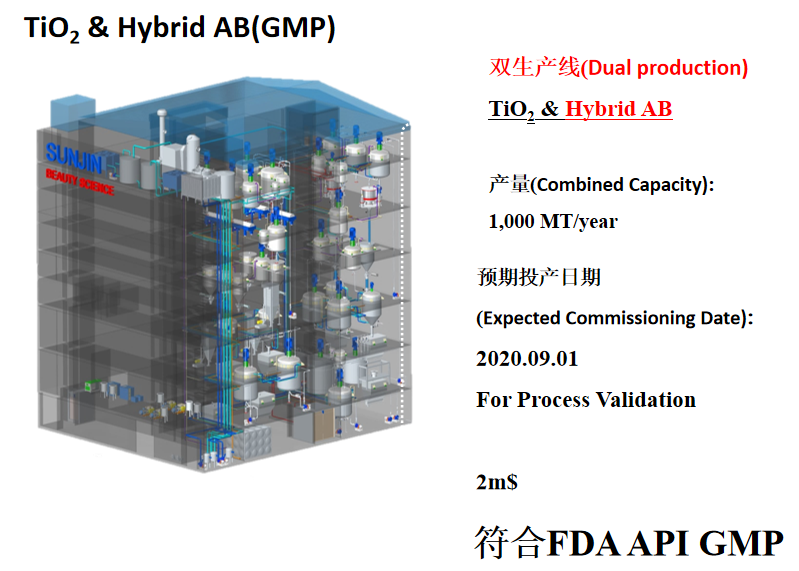
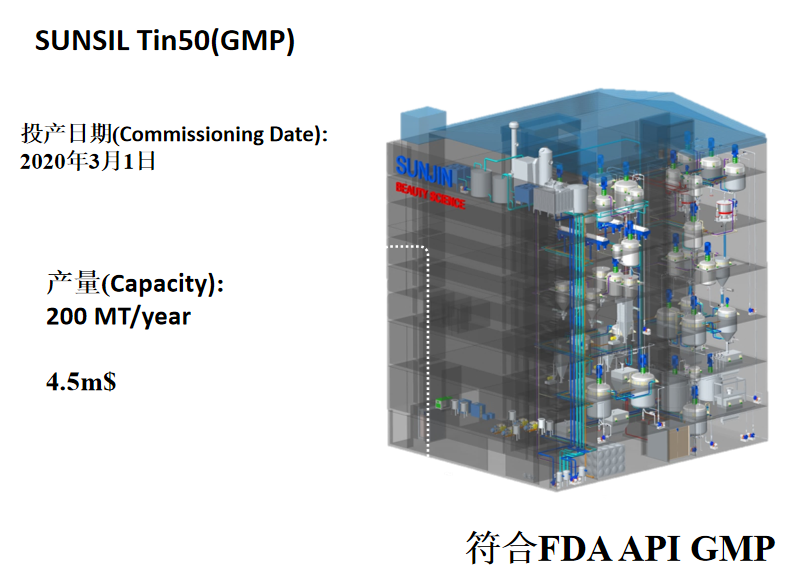
SUNJIIN 非纳米 TiO2 & ZnO 列在了National Drug Code.
SUNJIIN Non-Nano TiO2 and ZnO are listed in the National Drug Code.
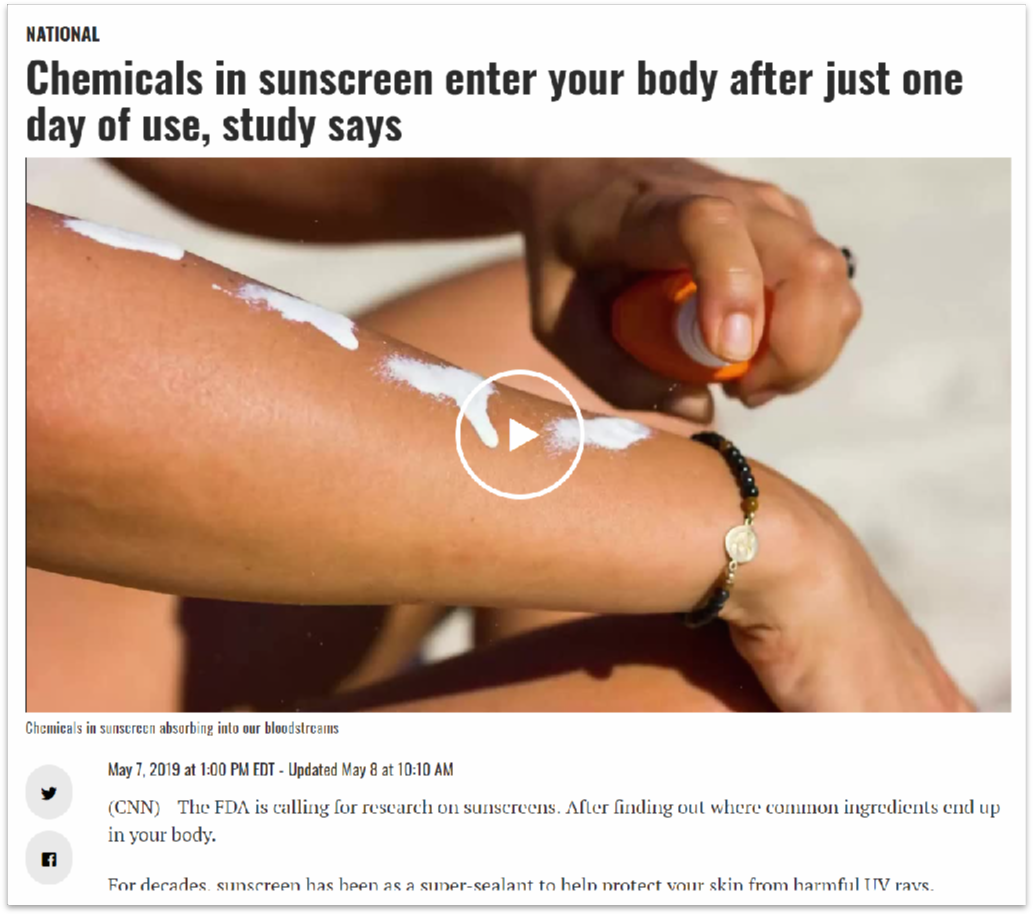
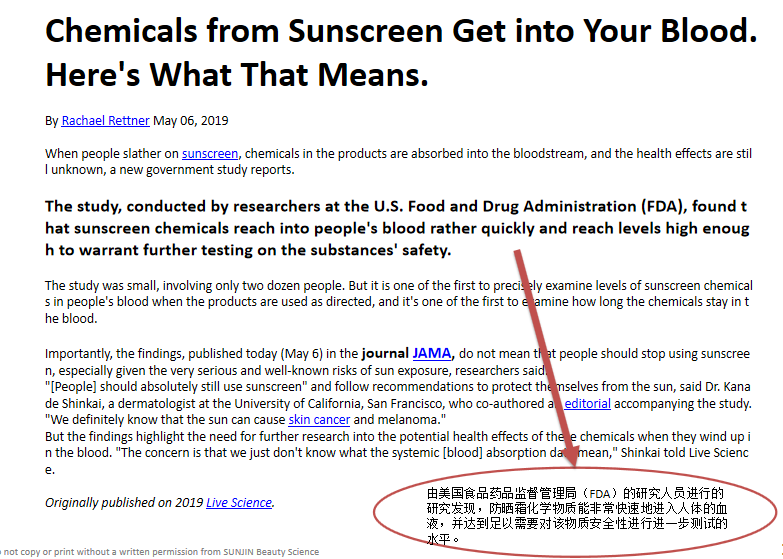
JAMA: The Journal of the American Medical Association 美国医学会杂志 Impact factor影响因子51.273 (2018)


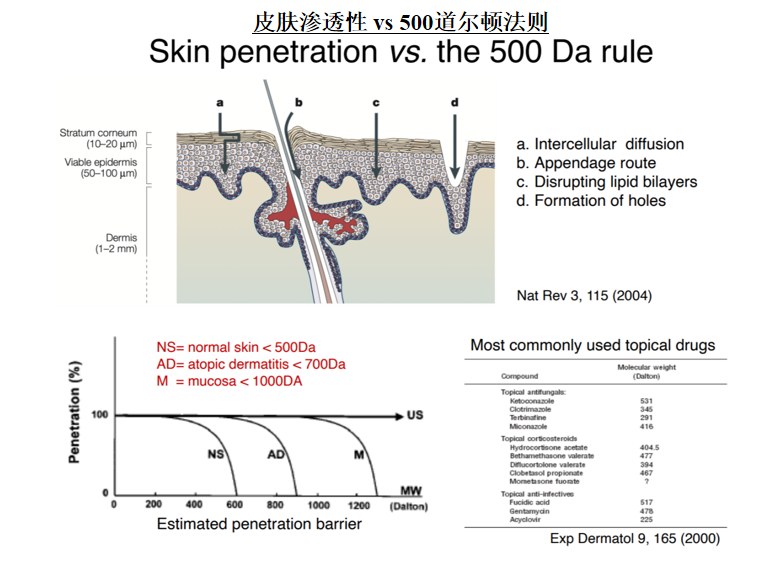
FDA的新建议: GRASE*
FDA’s New Proposal: GRASE*
针对美国的上市的防晒霜 for sunscreen in the U.S Feb 22, 2019
Proposes that, of the 16 currently marketed active ingredients, two ingredients – Zinc oxide and Titanium dioxide – are GRASE for use in sunscreens 氧化锌和二氧化钛-用在防晒霜中是GRASE(通常被认为是安全有效的) ; two ingredients – PABA and trolamine salicylate – are not GRASE for use in sunscreens due to safety issues. There are 12 ingredients for which there are insufficient safety data to make a positive GRASE determination at this time. To address these 12 ingredients, the FDA is asking industry and other interested parties for additional data.. *GRASE : Generally Recognized As Safe and Effective (通常被认为是安全有效的)
FDA 正在考虑将BEMT 纳入GRASE*
FDA的防晒霜专论(Sunscreen Monograph)中是否可以见到新成员?
Could the FDA Sunscreen Monograph See a New addition?
Aug 28, 2019
The U.S. Food and Drug Administration (FDA) has just published meeting notes in reference to a proposal by DSM Nutritional Products to add the sunscreen bemotrizinol (up to 10%) as a GRASE ingredient to the OTC Sunscreen Monograph. 在防晒霜中添加BEMT(不超过10%)认为是GRASE原料用于非处方(OTC)防晒专论。 In the official memorandum, the FDA calls for the submission of specific data to review within seven to nine months (hopefully), and will then issue a final order classifying bemotrizinol (also known as BEMT) as GRASE or not GRASE. *GRASE : Generally Recognized As Safe and Effective (通常被认为是安全有效的)
岛屿禁止令: 夏威夷( Hawaii)Island Ban: Hawaii
夏威夷禁止会伤害到珊瑚礁的防晒霜(Hawaii bans sunscreens that harm coral reefs) July 3, 2018
(CNN) — Hawaii Gov. David Ige on Tuesday signed the first bill in the country that will ban sunscreens containing chemicals harmful to coral reefs. The bill, which was passed by state lawmakers in May, will go into effect January 1, 2021 (2021年1月1日起生效). At that point, the sale or distribution of over-the-counter sunscreens containing oxybenzone (苯酮类) and octinoxate(甲氧基肉桂酸乙基己酯,OMC), which help filter UV rays, will be prohibited. A study by Haereticus Environmental Laboratory, a nonprofit scientific organization, found the chemicals cause bleaching, deformities, DNA damage and ultimately death in coral when they're washed off beachgoers or discharged into wastewater treatment plants and deposited into bodies of water
岛屿禁止令: 基韦斯特(Key West)Island Ban: Key West
基韦斯特加入夏威夷(Key West Joins Hawaii ) Feb 6, 2019
Experts predicted it, and now it is confirmed: the sunny beaches of the Florida Keys will no longer allow the use of sunscreens containing oxybenzone and octinoxate.
Does this mean California is next? 那是否意味着加州会是下一个?
According to a report by the Miami Herald, by a 6-1 vote, the Key West, Florida, City Commission banned the sale in the city limits of sunscreens that contain these ingredients. The ban will go into effect on Jan. 1, 2021. 该禁令将在2021年1月1日起生效。
越来越多的岛屿将加入执行防晒霜禁令(More islands will Enact the Sunscreen Ban)
全球范围(Worldwide)



Palau (January 2020) US Virgin Island (March 2020) Bonaire, Netherlands(January 2021)
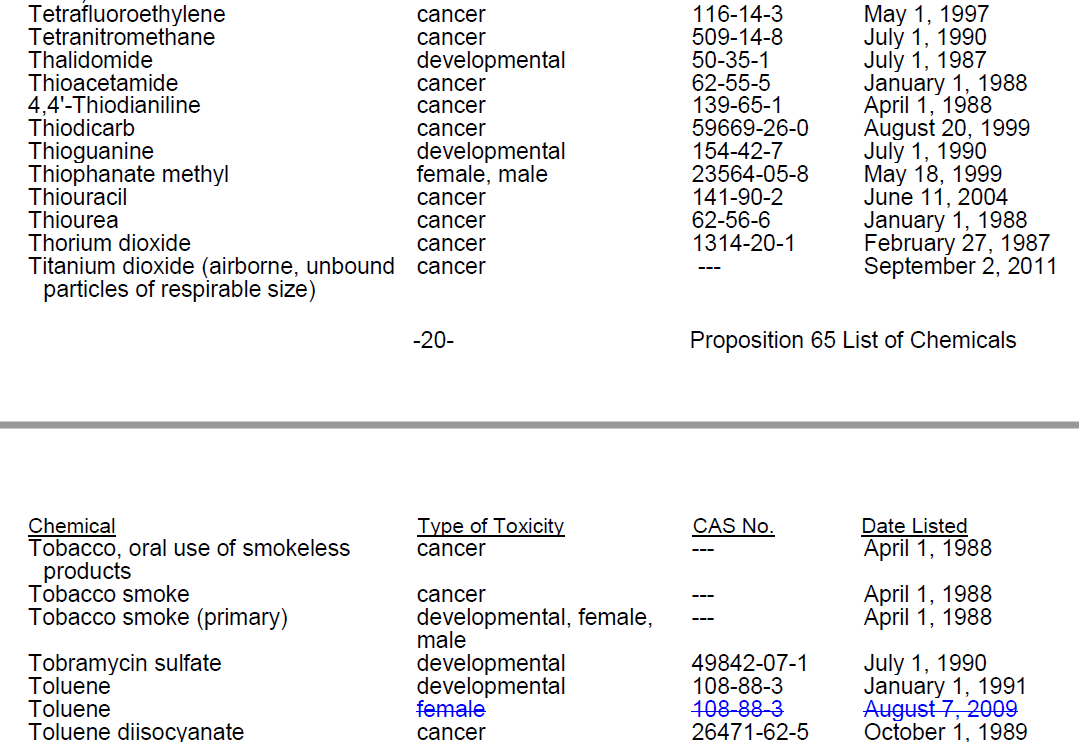
加利福尼亚州: 65号提案(California: Proposition 65)
65号提案清单(Proposition 65 List)